Did you know? The Moon has an Atmosphere
It is a common misconception that the Moon’s surface is a perfect vacuum. It is not! The Moon indeed has an atmosphere, a very tenuous one however. If you could capture the Moon’s entire atmosphere, it would weigh about 10,000 kg. In other words, it would weigh less than a large truck.
Sources of the lunar atmosphere
There are two main sources of the lunar atmosphere. One of them is outgassing, which is the release of gasses that originate from radioactive decay processes deep inside the crust and mantle of the Moon.
The second source is through a process known as sputtering: atoms are ejected from solid materials on the lunar surface due to the bombardment by energetic particles. The planet Mercury obtains its tenuous atmosphere in the same way.
Another minor source of the lunar atmosphere consists of leaks from space suits and the lunar habitation structures from the Apollo missions, as well as the gases ejected from the rockets during landing and liftoff. While it sounds nice that Humans contributed to the lunar atmosphere, most of it has probably been lost to space since then.
Losses of the lunar atmosphere
The Moon loses most of its atmosphere to space; however it is replenished at about the same rate, so that the total mass of the atmosphere remains relatively constant on average.
Some of the gases released by sputtering will be re-implanted into the lunar regolith due to the lunar gravity.
Also some of the atmosphere is lost to space either by solar radiation pressure, or if the gasses are ionized, they are swept away in the solar wind’s magnetic field.
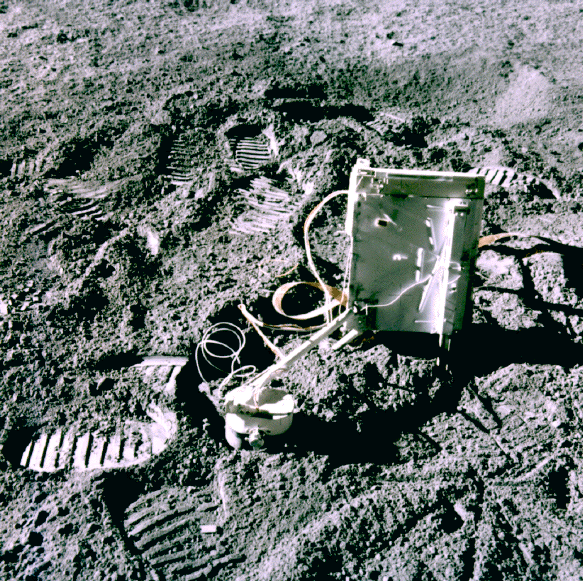 Device used by the Apollo astronauts to detect the lunar atmosphere. Photo by NASA.
Device used by the Apollo astronauts to detect the lunar atmosphere. Photo by NASA.
Composition of the lunar atmosphere
The average daytime lunar atmosphere consists of the following elements (proportions in atoms per cubic centimeter):
- Argon (40,000),
- Helium (2,000 – 40,000),
- Sodium (70),
- Potassium (17),
- Hydrogen (less than 17).
Other elements were also detected in much lower quantities. Radon-222 and polonium-210 was inferred from the data obtained by the Lunar Prospector alpha particle spectrometer. Argon-40, helium-4, oxygen, nitrogen and carbon were detected by devices used by the Apollo astronauts. One such device, called the “cold cathode gauge” is pictured above.
Some of those atoms would bind into molecules such as methane, carbon monoxide and carbon dioxide, since most elements cannot remain stable as single atoms.
In total, the lunar atmosphere consists of about 80,000 atoms per cubic centimeter, slightly more than what is believed to be the size of the atmosphere on Mercury.
Conclusion
Even though it has been scientifically proven that the Moon does indeed have a tenuous atmosphere, for all practical purposes the lunar environment is considered to be a near-perfect vacuum.
Would you like to receive similar articles by email?





8 Comments
Ajay
Each atom leaves a trace in the reflected light we observe from the Moon (or any other planet, or other celestial body).
Paul Tomaszewski
Thanks! 🙂
Jaquelyn Aredondo
Good stuff, loved this read!
Paul Tomaszewski
The Moon’s tectonic activity is dead now and has been for millions of years, but there is still some geological activity in the mantle and the core of the Moon.
P. I.
There also are more factors that come into play with an object like the Moon other than just relative gravity. The Moon’s small gravity and relative flatness doesn’t hold up well against forces like the solar wind. Also the Moon cooled off very quickly so its geological processes have been dead for a long time.
Paul Tomaszewski
As for the water it still needs confirmation to be absolutely sure, even though the evidence is compelling. Basically we would need to go there and see for ourselves.
Paul Tomaszewski
We know for sure, because we have sent a dozen people to the Moon! 😛
They made some tests while they were there.
Also it’s possible to detect atmospheres with a powerful telescope. Each atom leaves a trace in the reflected light we observe from the Moon (or any other planet, or other celestial body).
Mike
So very interesting and even still how they know for sure and have found water on the moon now. In the very bottom of dark canyons and craters on the moon that are never touched by sunlight. It’s water that’s got there from other planets and meteors hitting the moon and freezing there forever basically.